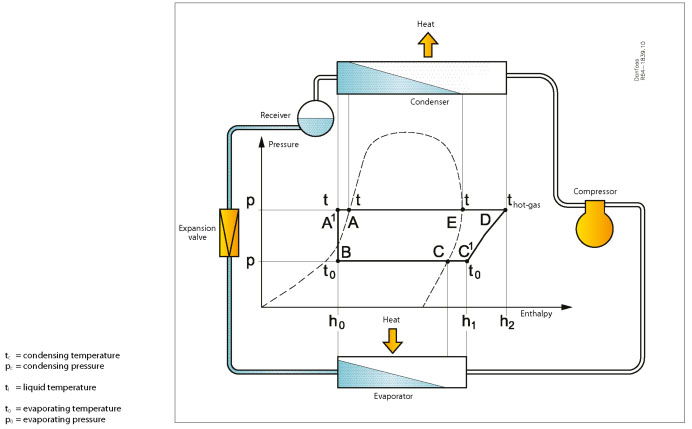
Refrigeration process pressure-enthalpy diagram
The condensed refrigerant in the condenser in a state that lies on the line for the boiling point of the liquid. Liquid, thus, the temperature tc, pressure pc also called saturated temperature and pressure. The condensed liquid in the condenser is cooled additionally in the refrigerator up to a temperature of A1 and now has a temperature tl and enthalpy of h0. Liquid now sub-cooled which means that it is cooled to a temperature below the saturation temperature.
The condensed liquid into the receiver is in a state of A1, which is a sub-cooling liquid. This fluid temperature may change if the receiver and liquid either heated or cooled to ambient temperature. If the liquid is cooled in hypothermia will increase, and Vice versa.
When the liquid passes through an expansion valve, its status changes from A1 to B. conditional change is caused by the boiling liquid due to a drop in pressure p0. At the same time a lower boiling point is, t0, due to pressure drop. The expansion valve enthalpy is a constant h0 and heat are not applied or removed.
On the evaporator, point B, there is a mixture of liquid and vapor, while in the evaporator at C vapour.
Evaporator point C1 is superheated steam, which means that the suction gas is heated to a higher temperature than the saturation temperature. Pressure and temperature are the same in point B, and at the exit point C1, where the gas is heated evaporator has absorbed the heat from the environment and the enthalpy has changed in comparison with 1 half-year.
When the refrigerant passes through the compressor its status changes from C1 to D. Pressure rises condensing pressure pc. The temperature rises to Thoth-gas, which is higher than the temperature of condensation of tc, because the couple was strongly overheated. More energy from the electric motor in the form of heat, has also been introduced and, consequently, the change of enthalpy h2.
Condenser inlet point D, condition, thus, one of the superheated steam at a pressure of PC. Heat is radiated from the condenser to the surroundings so that enthalpy again the changes in the primary point of A1. The first in the condenser occurs conditional change from a highly superheated steam to the saturated vapor (point E), then the condensation of saturated vapors. From point E to point A temperature (dew point) remains unchanged, the condensation and evaporation occurs at a constant temperature.
From point A to point A1 in the condenser of the condensed liquid further cooled down, but the pressure remains the same, and the liquid is now sub-cooled.
 .. ..
|

 ..
..